Ohio farmers working to improve water quality
Research being done at Blanchard River Demonstration Farm sites throughout Ohio is helping researchers determine which conservation practices work best for reducing nutrient and sediment loss. This information will help show farmers what tools and practices they can implement on their farms to improve agriculture’s impact on downstream water quality in Ohio.
New Economic Spreadsheet Tools
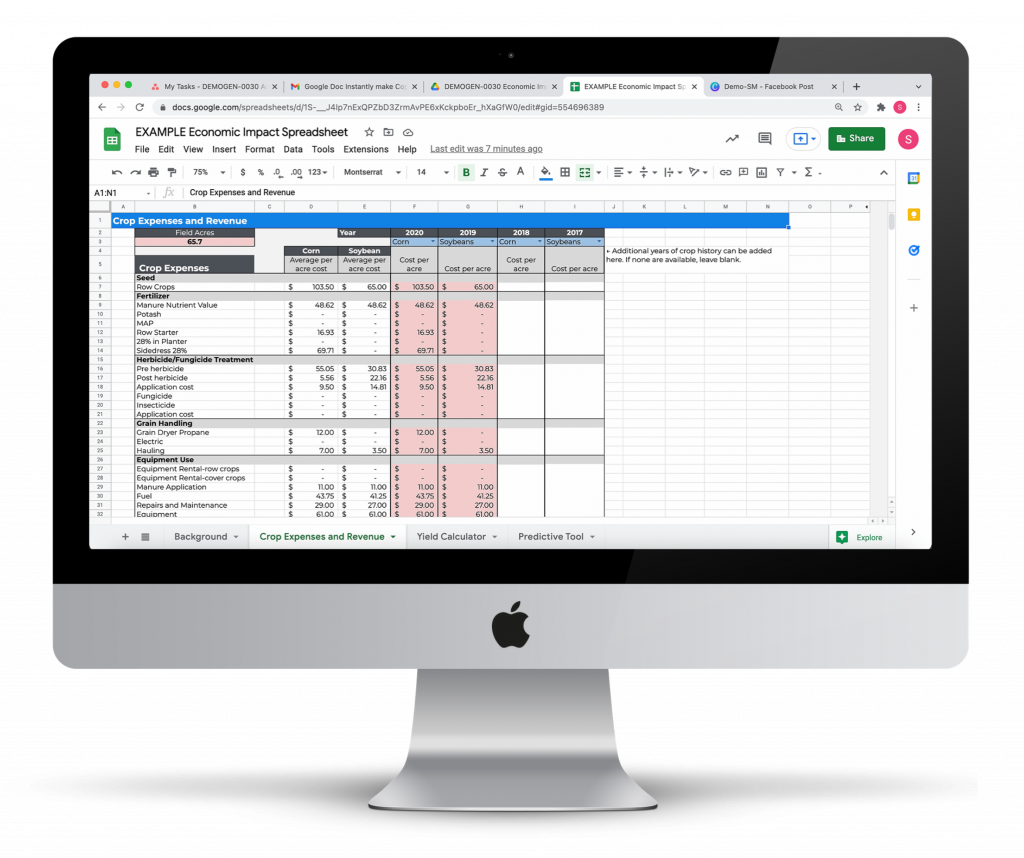
Conservation Practice Economic Impact Calculator
Evaluate the economic impact of installing a wetland or other land conservation program.
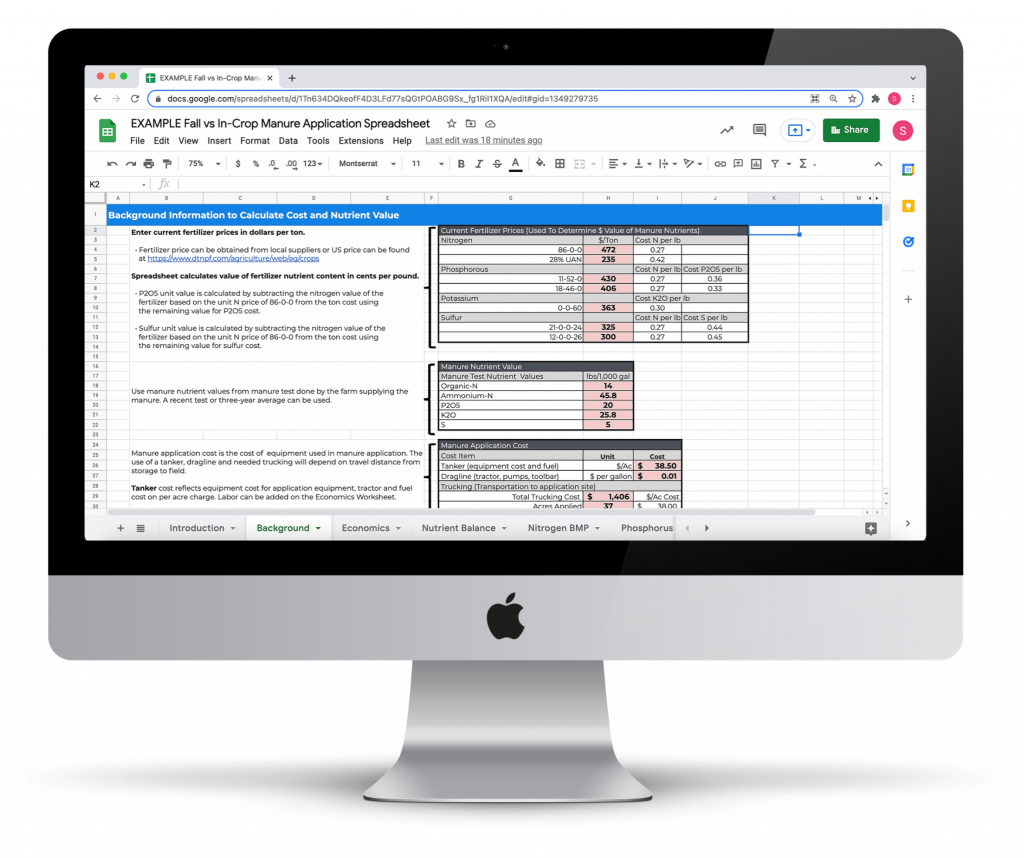
Fall vs In-Crop Manure Application Economic Return Calculator
Compare the economic return of liquid manure applied in the fall versus an in-crop application to standing corn.
Conservation practices improve the agricultural impact on downstream water quality in Ohio.
Our research helps develop better conservation practices.
Research being done at the Blanchard River Demonstration Farms and other related sites around the state is helping researchers determine what practices work best for reducing nutrient and sediment loss. Over the last five years, on-farm research has shown that three practices in particular help reduce nutrient and sediment loss:
1Following the 4R approach
2Reducing soil erosion
3Developing a water
management plan
1
Right Source
Right Rate
Right Time

Right Place
2
Reducing Soil Erosion
3
Developing Water Plan
Objectives, Mission & Initiatives
The Blanchard River Demonstration Farms Network (BRDFN) is a joint partnership between the U.S. Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS) and the Ohio Farm Bureau Federation, and is a Great Lakes Restoration Initiative project. The Demonstration Farms is a project showcasing and demonstrating conservation practices that improve agriculture’s impact on downstream water quality in Ohio.
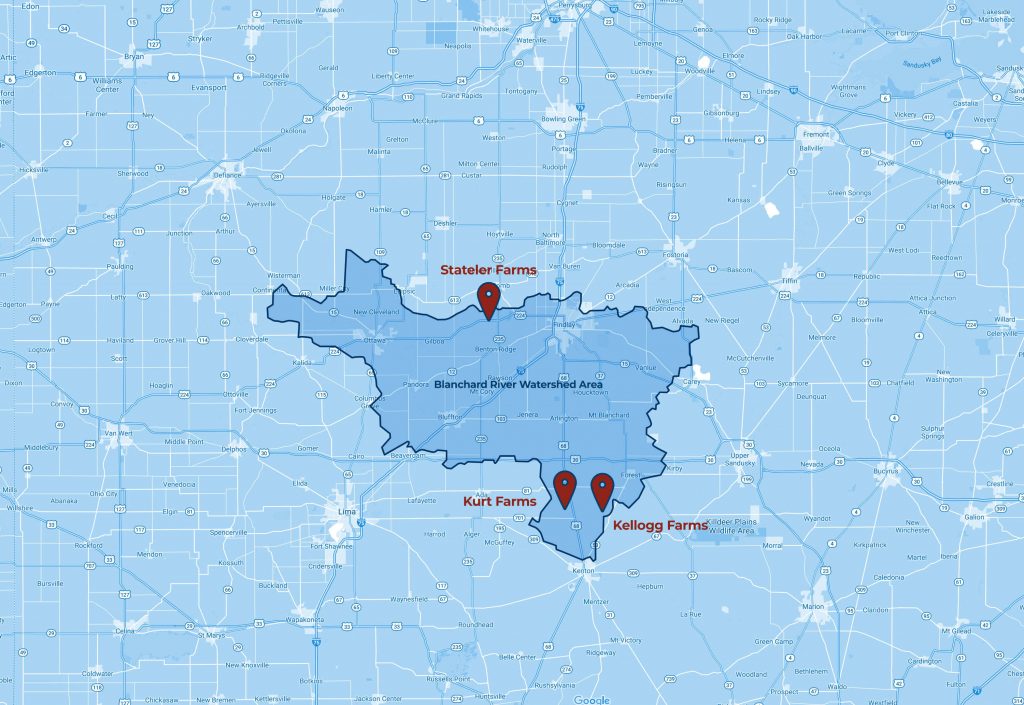
Meet Our Farmers
McComb, Ohio
1,000 acres corn, soybeans and wheat
7,200-head swine operation
9 conservation practices
Partners of the Blanchard River Demonstration Farms Network aim to help show farmers what tools and practices they can implement on their farms to limit their impact on downstream water quality. With their support, and through the implementation of conservation practices, agriculture can be part of the solution to a healthier Lake Erie.
News & Blog Stories
Upcoming Events
Podcast Episodes
Ep. 31: Indigo Ag
On this episode of Field Day with Jordan Hoewischer, we talk with Mike Thompson and Bryan Randell from Indigo Ag on their carbon programs available for farmers across the state. This episode is the fourth in a series that will help understand the breadth and depth of carbon program options for Ohio farmers.
Ep. 30: NORI Inc
On this episode of Field Day with Jordan Hoewischer, we talk with Aldyen Donnelly, co-founder of Nori Inc. about all things carbon and what programming Nori offers for farmers interested in carbon sequestration. This episode is the third in a series that will help understand the breadth and depth of carbon program options for Ohio farmers.
Ready to learn more?
















